Characteristics of Water
Water has three characteristics, i.e. physical, chemical and biological characteristics. The raw treated water can be checked and analysed by studying and testing these characteristics as below:
Physical Characteristics of Water
1. Colour
The presence of colour in water is not objectionable from health point of view, but may spoil the colour of the clothes being washed. The standard unit of colour is that which is produced by one milligram of platinum cobalt dissolved in one litre of distilled water.For public supplies, the colour number on cobalt scale should not exceed 20 and should be preferably less than 10.
Colour determined by an instrument is known as tintometer.
2. Turbidity of Water
The turbidity is measured by a turbidity rod or by a turbidity meter with optical observations and is expressed as the amount of suspended matter in mg/l or parts per million (ppm).
For water, ppm and mg/l are approximately equal.
The standard unit is that which is produced by one milligram of finely divided silica (fuller’s earth) in one litre of distilled water.
Turbidity Meters
Turbidity Rod:
The turbidity can be easily measured in the field with the help of a turbidity rod. It consists of an aluminium rod which is graduated as to give turbidity directly in silica units (mg/l)
Turbidimeter:
The turbidity can be easily measured in the laboratory with the help of a instruments called turbidity meter. In general, a turbidity meter works on the principle of measuring the interference caused by the water sample to the passage of light rays.
Jackson’s candle Turbidimeter:
The height of water column will therefore be more for less turbid water and vice versa. Longer the light path lower the turbidity. Such a turbidimeter can not measure turbidites lower than 25 JTU.
It can be used for natural sources only and can not be used to measure the turbidity of treated water supplies, for which Baylis turbidity meter or modern nephelometers are used.
Baylis Turbidimeters
One of the two glass tubes is filled with water sample (whose turbidity I to be measured) and the other is filled with standard water solution of known turbidity. The electric bulb is lighted and the blue colour in both the tubes is observed from the top of the instrument.
Modern Nephelometer: for low turbidity less than 1 unit.
NTU – Nephelometric Turbidity Units
FTU – Formazin Turbidity Units
Ratio turbidimeter: River water has maximum amount of turbidity.
3. Taste and Odour
The extent of taste or odour present in a particular sample of water is measured by a term called odour intensity, which is related with the threshold odour or threshold odour number.
Water to be tested is therefore gradually diluted with odour free water, and the mixture at which the detection of odour by human observation is just lost, is determined. The number of times the sample is diluted represents the threshold odour number.
For public supplies, the water should generally free from odour, i.e. the threshold number should be 1 and should never exceed 3.
4. Temperature of Water
For potable water, temperature of about about 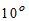 C is desirable. It should not be more than
C is desirable. It should not be more than 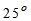 C.
C.
5. Specific Conductivity
The total amount of dissolved salts present in water can be easily estimated by measuring the specific conductivity of water.
Chemical Characteristics of Water
1. Total Solids and Suspended Solids
Total solids (suspended solids + dissolved solids) can be obtained by evaporating a sample of water and weighing the dry residue left and weighing the residue left on the filter paper.
The suspended solid can be found by filtering the water sample. Total permissible amount of solids in water is generally limited to 500 ppm.
2. pH value of Water
If 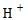 concentration increases, pH decreases and then it will be acidic.
concentration increases, pH decreases and then it will be acidic.
If ![clip_image008[1] clip_image008[1]](https://theconstructor.org/wp-content/uploads/2010/10/clip_image00813.jpg) concentration decreases, pH increases and then it will be alkaline.
concentration decreases, pH increases and then it will be alkaline.
pH + pOH = 14
if the pH of water is more than 7, it will be alkaline and if it is less than 7, it will be acidic.
The alkalinity is caused by the presence of bicarbonate of calcium and magnesium or by the carbonates of hydroxides of sodium, potassium, calcium and magnesium.
Some, but not all of the compounds that cause alkalinity also cause hardness.
pH Measurement:
the pH value of water can be measured quickly and automatically with the help of a Potentiometer.
The pH can also be measured by indicators as given below:
| Indicator | pH range of indicator dye | Original color | Final color produced |
| Methyl orange | 2.8 – 4.4 | Red | Yellow |
| Methyl red | 4.4 – 6.2 | Red | Yellow |
| Phenol red | 6.8 – 8.4 | Yellow | Red |
| Phenolphthalein | 8.6 – 10.3 | Yellow | Red |
Permissible pH value for public supplies may range between 6.6 to 8.4.
The lower value of pH may cause incrustation, sediment deposits, difficulty in chlorination.
3. Hardness of Water
Hard waters are undesirable because they may lead to greater soap consumption, scaling of boilers, causing corrosion and incrustation of pipes, making food tasteless etc.
Temporary Hardness: If bicarbonates and carbonates of calcium and magnesium are present in water, the water is render hard temporarily as this hardness can be removed to some extent by simple boiling or to full extent by adding lime to water. Such a hardness is known as temporary hardness or carbonate hardness.
Permanent Hardness: If sulphates, chlorides and nitrates of calcium or magnesium are present in water, they can not be removed at al by simple boiling and therefore, such water require special treatment for softening. Such a hardness is known as permanent hardness or non-carbonate hardness.
It is caused by sulphates, chlorides, nitrates of Ca and Mg.
Carbonate hardness = Total hardness or Alkalinity (which ever is less)
Non-carbonate hardness = Total hardness – Alkalinity
- Carbonate hardness is equal to the total hardness or alkalinity which ever is less
- Non-carbonate hardness is the total hardness in excess of the alkalinity. If the alkalinity is equal to or greater than the total hardness, there is no non-carbonate hardness.
- One French degree of hardness is equal to 10mg/l of CaCO3.
- One British degree of hardness is equal to a hardness of 14.25mg/l.
- Water with hardness upto 75 ppm are considered soft and above 200 ppm are considered hard and in between is considered as moderately hard.
- Underground waters are generally harder than surface waters.
- The prescribed hardness limit for public supplies range between 75 to 115 ppm.
4. Chloride Content
The chloride content of treated water to be supplied to the public should not exceed a value of about 250 ppm.
The chloride content of water can be measured by titrating the water with standard silver nitrate solution using potassium chromate as indicator.
(5) Nitrogen Content
The presence of nitrogen in water may occur in one or more of the following reasons:
- Free ammonia: It indicates very first stage of decomposition of organic matter. It should not exceed 0.15mg/l
- Albuminous or Organic Matter: It indicates the quantity of nitrogen present in water before the decomposition of organic molten has started. It should not exceed 0.3mg/l
- Nitrites: Not fully oxidized organic matter in water.
- Nitrates: It indicates fully oxidized organic matter in water (representing old pollution).
- Nitrites is highly dangerous and therefore the permissible amount of nitrites in water should be nil.
- Ammonia nitrogen + organic nitrogen = kjeldahl nitrogen
- Nitrates in water is not harmful. However the presence of too much of nitrates in water may adversely affect the health of infants causing a disease called methemoglobinemia commonly called blue baby disease.
- The nitrate concentration in domestic water supplies is limited to 45 mg/l.
6. Metal and other chemical substances in water:
Iron – 0.3ppm, excess of these cause discolouration of clothes.
Manganese – 0.05ppm
Copper – 1.3ppm
Sulphate – 250 ppm
Fluoride – 1.5 ppm, excess of this effects human lungs and other respiratory organs.
Fluoride concentration of less than 0.8 – 1.0 ppm cause dental cavity (tooth decay). If fluoride concentration is greater than 1.5ppm, causing spotting and discolouration of teeth (a disease called fluorosis).
7. Dissolved gases
Oxygen gas is generally absorbed by water from the atmosphere but it being consumed by unstable organic matter for their oxidation. Hence, if the oxygen present in water is found o be less than its saturation level, it indicates presence of organic matter and consequently making the waters suspicious.
Biological Oxygen Demand (BOD):
The extent of organic matter present in water sample can be estimated by supplying oxygen to this sample and finding the oxygen consumed by the organic matter present in water. This oxygen demand is known as Biological oxygen demand (BOD).
It is not practically possible to determine ultimate oxygen demand. Hence, BOD of water during the first five days at 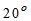 C is generally taken as the standard demand.
C is generally taken as the standard demand.
The BOD of safe drinking water must be nil.
Biological Characteristics of Water
Classification of bacteria based on oxygen requirement:
- Aerobic bacteria: Those which require oxygen for their survival.
- Anaerobic bacteria: Those which flourish in the absence of free oxygen.
- Facultative bacteria: Those which can survive with or without free oxygen.
Pathogenic bacteria
These can be tested and counted in the laboratories but with great difficulty. These tests are therefore, generally not performed in routine to check up of the water quality. The usual routine tests are generally conducted to detect and count the presence of coliforms which in themselves harmless organisms, but their presence or absence indicates the presence or absence of pathogenic bacteria.
Methods to measure the presence of coliform bacteria:
- Membrane filter technique (modern technique)
- Total Count Test
- Bacteria Coil (B-coil or E-coil Test)
- Most probable number (MPN)


Garage Door Repair Greenfield
ReplyDelete